Site Map
© 2024 PuraCap® Pharmaceutical LLC
All Rights Reserved.
This site is only intended for residents of the United States. The products discussed on this website may have different labeling in different countries
PuraCap® Pharmaceutical LLC,
100 Somerset Corp Blvd, Ste 3000
Bridgewater, NJ 08807
Phone: 908.941.5456
Fax: 908.941.5457

Contract Manufacturing
PuraCap offers full service contract manufacturing services for your products. From concept to commercilazation, we can provide you with full turn-key commercial development services.
With full capabilities to develop Monographed OTC’s, ANDA’s, 505(b) (1) and 505(b) (2), we can provide quaility, reliablity and cost effective manufacturing and development services. Our R & D teams in New Jersey & Wuhan, China will work with you every step of the way, to bring your current or future project to full commercializaton.
Our state of the art 140,000 sq ft soft gel facility in Wuhan China meets all cGMP FDA regulatory requirements for pharmaceutical (Rx & OTC’s) development and manufacturing. With capacity of up the 4 billion soft gel capsules annually we have the ability to meet your needs. For more information please contact us at bd@puracap.com






Manufacturing A World of Wellness
Global expertise in product development; producing quality, affordable pharmaceutical and healthcare products.
Click here to join our community
homeHOME











All PuraCap Pharmaceutical prescription
brand products meet our rigorous standards
for quality, accessibility and ease of therapeutic delivery.
PuraCap Laboratories develops & markets prescription generic medications to meet patient needs for quality, efficacy and affordable products.
Our over the counter (OTC) business, Puravation Pharmaceuticals provides many top quality, OTC store brand &
private label products.
With our turn-key services, we can develop Prescription and OTC soft gelatin capsules in our state of the art facility, designed and constructed in accordance with all US FDA cGMP regulatory guidances.









November 16, 2015
South Plainfield, NJ– November 3, 2015 – PuraCap Pharmaceutical LLC
announced today the introduction of a
new product, EpiCeram-L™ Lip Care,
a unique lip care formulation with an
exclusive delivery
July 10, 2014
PuraCap™ Pharmaceutical Introduces
Unique EpiCeram® 225g Airless Pump
EpiCeram® Controlled Release Skin
Barrier Emulsion Now Offers Dosing
Flexibility with 2 Sizes.
November 13, 2015
South Plainfield, NJ–November 11, 2015 – PuraCap Pharmaceutical LLC. is pleased to announce that the US Food and Drug Administration (FDA) has completed a successful inspection of its affiliated
May 19, 2014
Max Baucus, US Ambassador to China visits Wuhan Softgel Capsule Manufacturing Plant in Wuhan China
SOUTH PLAINFIELD, N.J., May 19, 2014 /PRNewswire/ — PuraCap Pharmaceutical today announced


Site Map
© 2024 PuraCap® Pharmaceutical LLC
All Rights Reserved.
This site is only intended for residents of the United States. The products discussed on this website may have different labeling in different countries
PuraCap® Pharmaceutical LLC,
100 Somerset Corp Blvd, Ste 3000
Bridgewater, NJ 08807
Phone: 908.941.5456
Fax: 908.941.5457


Contract Manufacturing
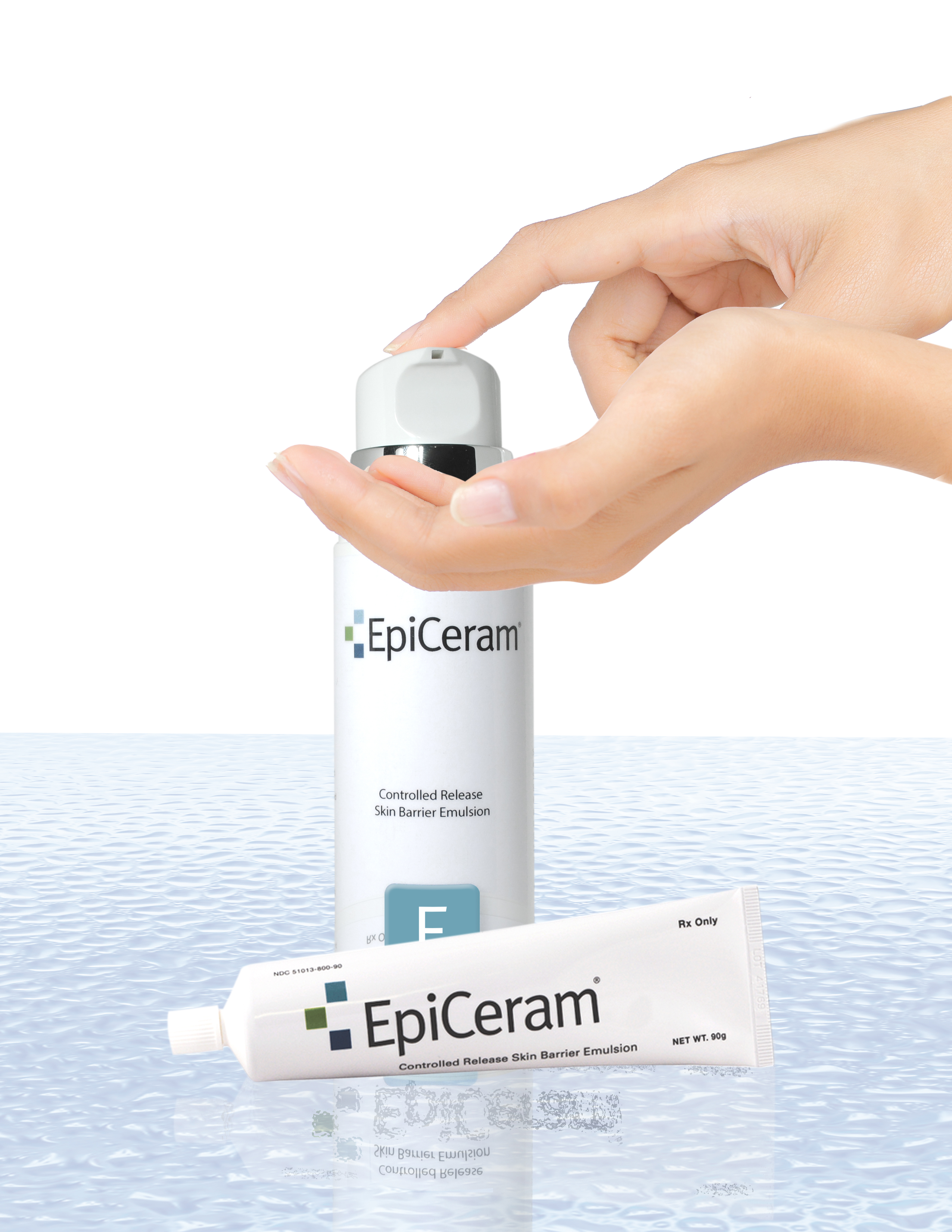
EpiCeram® is a leading prescription product that has the ability to help repair and heal the skin barrier through a unique mode-of-action different from other medications and eczema treatments. Typically, EpiCeram® is prescribed along with a topical steroid product, but EpiCeram® can also be prescribed alone. Only EpiCeram® contains the skin’s natural level of 3 essential lipids, such as ceramides, cholesterol and free fatty acids, which are reduced in patients with eczema or atopic dermatitis. EpiCeram® is steroid-free, fragrance-free, noncomedogenic1 , paraben-free, and propylene glycol-free.
EpiCeram-L™ Lip Care is the first of its kind, using a patented MultiSal™ Technology an exclusive multi-component controlled-release of important lipids, creating a moist, luxuriant feel.
EpiCeram-L™ Lip Care contains 3 essential lipids ceramides, conjugated linoleic acid (CLA) and cholesterol.
EpiCeram-L™ Lip Care is steroid-free, paraben-free, gluten-free, petrolatum-free, fragrance-free, color-free, and aloe rich to soothe lips on contact. It is ideal for the most severe, dry, cracked lips.




PuraCap offers full service contract manufacturing services for your products. From concept to commercilazation, we can provide you with full turn-key commercial development services.
With full capabilities to develop Monographed OTC’s, ANDA’s, 505(b) (1) and 505(b) (2), we can provide quaility, reliablity and cost effective manufacturing and development services. Our R & D teams in New Jersey & Wuhan, China will work with you every step of the way, to bring your current or future project to full commercializaton.
Our state of the art 140,000 sq ft soft gel facility in Wuhan China meets all cGMP FDA regulatory requirements for pharmaceutical (Rx & OTC’s) development and manufacturing. With capacity of up the 4 billion soft gel capsules annually we have the ability to meet your needs. For more information please contact us at bd@puracap.com
A wide range of PuraCap products are manufactured by our affiliated manufacturing partner, Humanwell Puracap Pharmaceuticals (Wuhan) Co., Ltd, a US FDA cGMP compliant facility that complies with international regulatory standards. Our goal is to put quality first, by building quality through design and a commitment to the highest standards. We strive to exceed USFDA, cGMP standards to assure our customers the quality product they expect.
The experienced manufacturing team, led by a well-recognized technical manufacturing authority in the soft gelatin pharmaceutical capsule industry, is committed to growing our business responsibly – utilizing proven and innovative processes and procedures to produce quality products without compromise. The manufacturing team is backed by a U.S. executive team with decades of experience in FDA (Federal Drug Administration) regulated prescription drug cGMP quality system management and process validation.
The Manufacturing team strives to provide cost-efficiency through manufacture at state-of-the art facilities built to USFDA specifications. Our model for success is to make use of labor as well as manufacturing efficiencies while meeting the highest quality standards. This model sets us apart from the competition. Our customers can be confident in our products—-highest quality at a competitive price.