Site Map
© 2024 PuraCap® Pharmaceutical LLC
All Rights Reserved.
This site is only intended for residents of the United States. The products discussed on this website may have different labeling in different countries
PuraCap® Pharmaceutical LLC,
100 Somerset Corp Blvd, Ste 300
Bridgewater, NJ 08807
Phone: 908.941.5456
Fax: 908.941.5457

News

Manufacturing A World of Wellness
Global expertise in product development; producing quality, affordable pharmaceutical and healthcare products.
Click here to join our community
homeHOME











All PuraCap Pharmaceutical prescription
brand products meet our rigorous standards
for quality, accessibility and ease of therapeutic delivery.
PuraCap Laboratories develops & markets prescription generic medications to meet patient needs for quality, efficacy and affordable products.
Our over the counter (OTC) business, Puravation Pharmaceuticals provides many top quality, OTC store brand &
private label products.
With our turn-key services, we can develop Prescription and OTC soft gelatin capsules in our state of the art facility, designed and constructed in accordance with all US FDA cGMP regulatory guidances.









November 16, 2015
South Plainfield, NJ– November 3, 2015 – PuraCap Pharmaceutical LLC
announced today the introduction of a
new product, EpiCeram-L™ Lip Care,
a unique lip care formulation with an
exclusive delivery
July 10, 2014
PuraCap™ Pharmaceutical Introduces
Unique EpiCeram® 225g Airless Pump
EpiCeram® Controlled Release Skin
Barrier Emulsion Now Offers Dosing
Flexibility with 2 Sizes.
November 13, 2015
South Plainfield, NJ–November 11, 2015 – PuraCap Pharmaceutical LLC. is pleased to announce that the US Food and Drug Administration (FDA) has completed a successful inspection of its affiliated
May 19, 2014
Max Baucus, US Ambassador to China visits Wuhan Softgel Capsule Manufacturing Plant in Wuhan China
SOUTH PLAINFIELD, N.J., May 19, 2014 /PRNewswire/ — PuraCap Pharmaceutical today announced


Site Map
© 2024 PuraCap® Pharmaceutical LLC
All Rights Reserved.
This site is only intended for residents of the United States. The products discussed on this website may have different labeling in different countries
PuraCap® Pharmaceutical LLC,
100 Somerset Corp Blvd, Ste 300
Bridgewater, NJ 08807
Phone: 908.941.5456
Fax: 908.941.5457


News
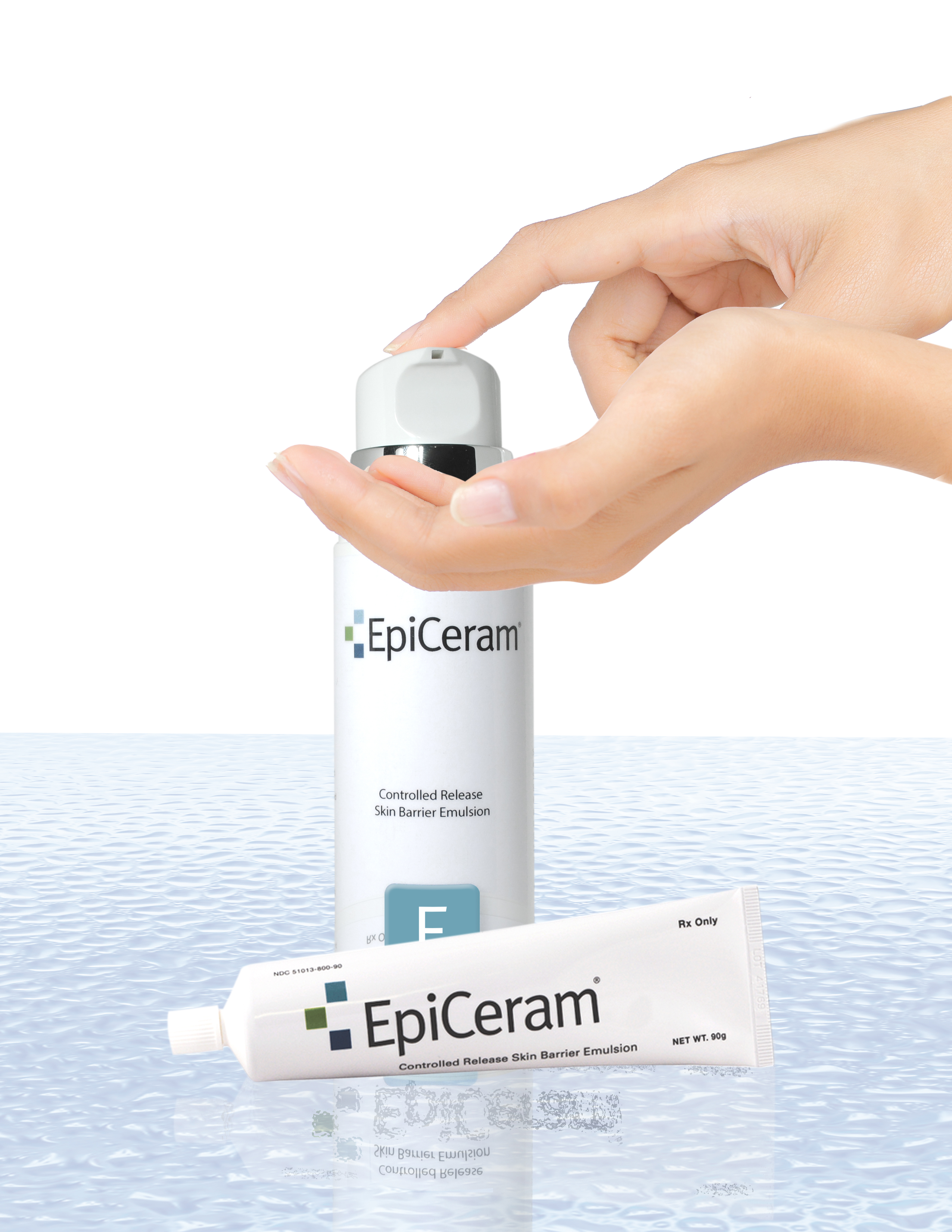
EpiCeram® is a leading prescription product that has the ability to help repair and heal the skin barrier through a unique mode-of-action different from other medications and eczema treatments. Typically, EpiCeram® is prescribed along with a topical steroid product, but EpiCeram® can also be prescribed alone. Only EpiCeram® contains the skin’s natural level of 3 essential lipids, such as ceramides, cholesterol and free fatty acids, which are reduced in patients with eczema or atopic dermatitis. EpiCeram® is steroid-free, fragrance-free, noncomedogenic1 , paraben-free, and propylene glycol-free.
EpiCeram-L™ Lip Care is the first of its kind, using a patented MultiSal™ Technology an exclusive multi-component controlled-release of important lipids, creating a moist, luxuriant feel.
EpiCeram-L™ Lip Care contains 3 essential lipids ceramides, conjugated linoleic acid (CLA) and cholesterol.
EpiCeram-L™ Lip Care is steroid-free, paraben-free, gluten-free, petrolatum-free, fragrance-free, color-free, and aloe rich to soothe lips on contact. It is ideal for the most severe, dry, cracked lips.




PuraCap Laboratories develops a wide range of prescription generic medications to meet patient needs for quality and affordability. With our own ANDA development, strategic partnerships, in-licensing agreements, we offer a wide range of high quality prescription generics.
A wide range of PuraCap products are manufactured in our affiliated manufacturing partner, Humanwell Puracap Pharmaceuticals (Wuhan) Co., Ltd, a US FDA cGMP compliant facility that comply with international regulatory standards. Our goal is to put quality first, by building quality through design and a commitment to the highest standards. We strive to exceed USFDA, cGMP standards to assure our customers the quality product they expect will be delivered.
Their experienced manufacturing team, led by a well-recognized technical manufacturing authority in the soft gelatin pharmaceutical capsule industry, is committed to growing our business responsibly – utilizing proven and innovative processes and procedures to produce quality products without compromise. Our manufacturing team is backed by a U.S. executive team with decades of experience in FDA (Federal Drug Administration) regulated prescription drug cGMP quality system management and process validation.
They strive to provide cost-efficiency through manufacture at state-of-the art facilities built to USFDA specifications. Our model for success is to make use of labor as well as manufacturing efficiencies while meeting the highest quality standards. This model sets us apart from the competition. Our customers can be confident in our products—-highest quality at a competitive price.











Our global presence in prescription brands, prescription generics, and Over the Counter (OTC) products, including private label products are growing. Our highly-skilled team meets market needs through innovation – introducing cost-efficient models to establish new product lines, utilizing global distribution networks and ensuring our products are made in world-class manufacturing facilities.
We explore ways to build expertise and expand offerings through organic growth and by seeking in-licensing opportunities, partnerships, acquisitions and other professional alliances.
- OUR VISION
To build a global pharmaceutical company renowned for affordable, world-class quality products grounded in scientific integrity.
- OUR MISSION
To enhance medication compliance and improve patient outcomes by bringing affordable, world-class quality pharmaceutical products to our customers.
- CORPORATE BROCHURE
Click to download
- OUR VISION
To build a global pharmaceutical company renowned for affordable, world-class quality products grounded in scientific integrity.
- OUR MISSION
To enhance medication compliance and improve patient outcomes by bringing affordable, world-class quality pharmaceutical products to our customers.
- CORPORATE BROCHURE
Click to download
Dahai Guo
Elise Klein
Mark Bolling
Founder and CEO of
PuraCap Pharmaceutical, LLC
General Manager,
Branded Rx Sales
Vice President,
Sales & Marketing, OTC Business
Xiaofeng Meng, PhD
Sean Weeks
Vice President
Vice President,
Global Manufacturing
Mahboob Rahman
Sr. Director R&D
Adam Feng
PuraCap® Pharmaceutical LLC introduces new EpiCeram-L™ Lip Care

Piscataway, NJ – November 3, 2015 – PuraCap Pharmaceutical LLC announced today the introduction of a new product, EpiCeram-L™ Lip Care, a unique lip care formulation with an exclusive delivery system for the treatment of dry lip conditions. PuraCap Pharmaceutical is the maker of EpiCeram® Controlled Release Skin Barrier Emulsion, a leading prescription product indicated for the treatment of dry skin conditions and management of burning and itching associated with various skin conditions including atopic dermatitis. The introduction of EpiCeram-L adds to PuraCap’s growing portfolio of dermatology products.
EpiCeram-L Lip Care is a unique lip care formulation with an exclusive delivery system developed specifically for severely chapped, dry, or cracked lips. EpiCeram-L Lip Care uses patented MultiSal™ technology to deliver 3 essential lipids – ceramides, free fatty acids and cholesterol in an all natural, steroid-free, paraben-free, fragrance-free, and color-free formulation. EpiCeram-L Lip Care is available exclusively through prescribing healthcare professionals.
“PuraCap has a strong, growing portfolio of dermatology products and EpiCeram-L Lip Care is an exciting new addition,” said Dahai Guo, CEO of PuraCap Pharmaceutical LLC. “We have listened to the needs of our customers and are pleased to introduce EpiCeram-L Lip Care.”
“Now there is a new option for patients who suffer from severely chapped, dry or cracked lips,” says Elise Klein, Vice President and General Manager of PuraCap’s Branded Rx division. “We look forward to continuing to meet the needs of our physicians and patients.” Physicians will be able to dispense EpiCeram-L directly to their patients from the office, which will make it very easy for patients to get the lip balm and begin treatment immediately. EpiCeram-L will be distributed through a patented web based ordering system. The program is designed to provide physicians easy access to PuraCap’s growing portfolio of dermatology products.
About PuraCap Pharmaceutical LLC
PuraCap Pharmaceutical LLC is an emerging, fully integrated pharmaceutical company with expertise in product development, manufacturing, and bringing affordable, world-class quality products to their customers. The PuraCap corporate structure supports a two-pronged approach for global growth with dedicated companies in the areas of prescription brands (PuraCap Pharmaceutical) as well as prescription generics and OTC and private label brands. PuraCap Pharmaceutical continues to innovate using our soft gel expertise and developing other soft gel options. Go to www.puracap.com for more information.
Forward-Looking Statement
All of the statements contained in this news release, other than statements of fact that are independently verifiable at the date hereof, are forward-looking statements. Such statements, based as they are on the current expectations of management, inherently involve numerous risks and uncertainties, known and unknown. Some examples of known risks are: the impact of general economic conditions, general conditions in the pharmaceutical industry, changes in the regulatory environment in the jurisdictions in which PuraCap Pharmaceutical LLC does business, fluctuations in costs, and changes to the competitive environment due to consolidation or otherwise. Consequently, actual future results may differ materially from the anticipated results expressed in the forward-looking statements.
Media Contact:
info@puracap.com
908-941-5456
PuraCap® Pharmaceutical Introduces Unique EpiCeram® 225g Airless Pump
EpiCeram® Controlled Release Skin Barrier Emulsion Now Offers Dosing Flexibility with 2 Sizes
South Plainfield, NJ – July 7, 2014 PuraCap™ Pharmaceutical today announced the newest addition to the EpiCeram® product line, the EpiCeram® 225g Airless Pump. The unique airless pump gives patients more EpiCeram® to treat any size area of atopic dermatitis. The special dispensing system allows for more control, delivering EpiCeram® consistently and precisely in a convenient to use, portable, pump action dispenser. The EpiCeram® Airless Pump also offers cost savings with BID dosing and a controlled release formulation that is long lasting.
“Our physicians have been asking for a larger size, so we responded with a uniquely elegant pump dispenser that holds 2 ½ times more EpiCeram® than our 90g tube,” said Elise Klein, VP of Brand Marketing for PuraCap Pharmaceutical. “Offering both 90g and 225g size gives the provider flexibility to prescribe an amount that is right for each patient’s needs.”
EpiCeram® Controlled Release Skin Barrier Emulsion is a prescription product that helps to relieve the burning and itching associated with skin conditions such as atopic dermatitis/eczema. It is the only topical agent formulated with three essential lipids (ceramides, free fatty acids, and cholesterol) in a physiologically balanced ratio, which mimics the lipid concentration found in the skin. In addition, EpiCeram® is designed with a unique, patented, controlled release technology that delivers 24-hour barrier repair benefits with just a twice-daily application. A steroid-free topical formulation, EpiCeram® is FDA-cleared for the treatment of atopic dermatitis. For more information on EpiCeram® visit www.epiceram-us.com.
About PuraCap™ Pharmaceutical LLC
PuraCap™ Pharmaceutical LLC is an emerging, fully integrated pharmaceutical company with expertise in product development, manufacturing and bringing affordable, world-class quality products to their customers. The PuraCap™ corporate structure supports their three-prong approach for global growth with dedicated companies in the areas of prescription brands, PuraCap™ Pharmaceutical LLC; prescription generics, PuraCap™ Laboratories Inc.; and OTC and Private Label products, PuraVation™ Pharmaceuticals Inc. For more information on PuraCap™ Pharmaceutical LLC, visit the company’s website at http://www.PuraCap.com.
Media Contact:
Elise Klein
Vice President and General Manager
Branded Rx Products
Elise.klein@puracappharma.com
908-941-5456 ext. 640

PuraCap® Pharmaceutical LLC Announces Acquisition of Blu Pharmaceuticals LLC and Blu Caribe

Purchase expands PuraCap’s presence in both US and Global Markets
South Plainfield, NJ –March 23, 2016 – Puracap Pharmaceutical LLC announced today the acquisition of Blu Pharmaceutical LLC and Blu Caribe Inc creating PuraCap International LLC, a joint venture between PuraCap and Dangdai International Group Co., Ltd. This acquisition will expand PuraCap’s world-class manufacturing expertise beyond soft gelatin capsules to include oral tablet & capsule dosage forms for the both the US and global markets.
Under Puracap International LLC, the newly created company will grow and strengthen PuraCap’s considerable manufacturing capabilities over the next several years, according to Puracap Pharmaceutical CEO Dahai Guo.
“This acquisition is a major step in the growth of our capability in the manufacturing of oral solid dosage forms in the United States, which complements our state-of-the-art soft gelatin capsule manufacturing facility in Wuhan, China” stated Mr. Guo.
The acquisition includes a state-of-the-art 145,000 sq. ft. FDA approved, cGMP compliant manufacturing facility in Dorado, Puerto Rico, as well as an 185,000 sq ft warehouse & distribution center in Franklin, Kentucky, USA.
“Dangdai International Group Co., Ltd is especially pleased to be part of this joint venture that broadens our investment activity into the western hemisphere in a company poised for strong growth” stated Dr. Hansheng Zhou , CEO of Wuhan Dangdai Science & Technology Industries (Group) Co., Ltd. Dangdai International Group Co. Ltd. is a wholly owned subsidiary of Wuhan Dangdai Science & Technology Industries (Group) Co. Ltd., a conglomerate with a substantial medical and pharmaceutical portfolio in China. Dr. Zhou also serves as the Chairman of the Board at the newly formed PuraCap International LLC.
“This acquisition brings us further infrastructure and sales channels, not only in the retail generic drug business, but also the US government purchasing tender business channel to help meet the growing demand for generic prescription products”, added Mr. Sean Weeks, President of PuraCap International LLC.
About PuraCap Pharmaceutical LLC
PuraCap Pharmaceutical LLC is an emerging, fully integrated pharmaceutical company with expertise in product development, manufacturing and distribution bringing, high quality products & services to their customers. PuraCap’s corporate structure supports a two-pronged approach for global growth in the areas of prescription brands (PuraCap Pharmaceutical LLC) as well as prescription generics and OTC and private label brands (PuraCap International LLC).
About Wuhan Dangdai Science & Technology Industries (Group) Co., Ltd.
Founded in 1988, Wuhan Dangdai Science & Technology Industries (Group) Co., Ltd is a China-based privately held conglomerate. Wuhan Dangdai’s business mission focuses on innovation, transparency, governance, prudence and social responsibility. Its portfolio is comprised of several Chinese publicly listed entities and private companies in North America and Africa. Wuhan Dangdai’s diverse enterprise includes biopharmaceuticals, media, entertainment, real estate development, tourism, agriculture, textile and financial services.
Forward-Looking Statement
All of the statements contained in this news release, other than statements of fact that are independently verifiable at the date hereof, are forward-looking statements. Such statements, based as they are on the current expectations of management, inherently involve numerous risks and uncertainties, known and unknown. Some examples of known risks are: the impact of general economic conditions, general conditions in the pharmaceutical industry, changes in the regulatory environment in the jurisdictions in which PuraCap Pharmaceutical LLC and Dangdai International Group Co., Ltd do business, fluctuations in costs, and changes to the competitive environment due to consolidation or otherwise. Consequently, actual future results may differ materially from the anticipated results expressed in the forward-looking statements.
Media Contact:
Sean Weeks
President
PuraCap International LLC
Sean.weeks@puracappharma.com
www.puracap.com
908-941-5456
From left to right: Sean Weeks, President of PuraCap International, Dr. Hansheng Zhou, CEO of Wuhan Dangdai Science & Technology Industries Group Co., Ltd., Ray Mulvey, Co-founder of Puracap Pharmaceutical, Dahai Guo, CEO of PuraCap Pharmaceutical, Bill Luster, Founder of Blu Pharmaceuticals, Sharon Luster, Executive Vice President of Blu Pharmaceuticals, and Elise Klein, VP General Manager Branded Rx Products of PuraCap Pharmaceutical.
Purchase Will Expand Humanwell’s and PuraCap’s US Generics platform
Piscataway, NJ – March 30, 2016 – Humanwell Healthcare Group and PuraCap Pharmaceutical LLC announced today that the companies have entered into a definitive agreement to acquire 100% of the membership interests of Epic Pharma, LLC of Laurelton, NY for $550 million. The acquisition will further establish Humanwell and PuraCap in the US generics market and expand their existing commercial and manufacturing capabilities.
The acquisition of Epic will provide a robust generic product portfolio which includes tablets, 2-piece capsules and powder dosage form products as well as a future product portfolio that will include a series of controlled drug substances. Humanwell and PuraCap will acquire Epic’s balanced and diversified portfolio, which currently consists of 15 marketed generic products and a pipeline of 37 products, and this acquisition will complement PuraCap’s own R&D efforts by utilizing Epic’s strengths in the manufacture and development of controlled drug substances and powder formulations. The Epic acquisition will also add a US FDA & DEA inspected GMP manufacturing footprint of 110,000 square feet in Laurelton, New York, and result in the addition of 215 employees to the Humanwell and PuraCap US Operations Team.

Humanwell Healthcare Group and PuraCap Pharmaceutical LLC To Acquire Epic Pharma, LLC
Richard Wang, the Chairman of Humanwell Healthcare Group, stated that “This acquisition continues to broaden our global investments consistent with our strategy for the creation of a truly global pharmaceutical company for Humanwell Healthcare Group”.
“This acquisition is a major step in the growth of our company. In addition to strong and growing OTC and Branded Business units in the US, this acquisition along with our recent acquisition of Blu Pharmaceutical LLC & Blu Carib Inc., firmly establishes Humanwell and PuraCap in the US Generic Rx Pharmaceutical segment. The PuraCap US footprint continues to expand our tablet, softgel, 2-piece capsules, cream, and powder product portfolio,” stated Dahai Guo, CEO of PuraCap and also President of Humanwell USA LLC. “The addition of Epic is an important addition to the PuraCap family. We look forward to it being a platform for the development of our generic pharmaceutical business, both in the USA as well as internationally”.
Under the terms of the agreement, the acquisition is subject to certain conditions, including the expiration of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act, obtaining approval of Humanwell stockholders, obtaining various filing notices and registrations from certain governmental entities in the People’s Republic of China, and other customary conditions. The parties expect the transaction to close in the second quarter of 2016.
China Merchants Bank New York Branch is the Bookrunner and Mandated Lead Arranger in this transaction. Nomura served as financial advisor and Fox Rothschild LLP served as U.S. legal counsel to Humanwell and PuraCap. Jefferies LLC served as financial advisor and Hughes Hubbard & Reed LLP served as U.S. legal counsel to Epic.
About Humanwell Healthcare Group
Humanwell Healthcare (Group) Co., Ltd is a fully integrated life science company that focuses on investing, developing and managing healthcare companies. It was founded in 1993 and has been listed on the Shanghai Stock Exchange since 1997 (ticker: 600079,SH). With a market capitalization of US$3.5 Billion, it is recommended for its top investment value by institutional investors.
Humanwell invests in companies who are leaders in anesthetics, CNS, reproductive biological medicines, herbal medicines, and diagnostics and medical devices in China. In 2015, Humanwell Healthcare Group achieved $1.6 billion in operating revenue, through both organic growth and expansion through acquisitions. Go to www.renfu.com.cn for more information.
About PuraCap Pharmaceutical LLC
PuraCap Pharmaceutical LLC is a New Jersey based fully integrated pharmaceutical company with expertise in product development, manufacturing, and bringing affordable, world-class quality products to their customers. The PuraCap corporate structure supports a three-pronged approach for global growth with dedicated companies in the areas of prescription brands (PuraCap Pharmaceutical) as well as prescription generics and OTC and private label brands (PuraCap International LLC). PuraCap continues to innovate using soft gel expertise developing other soft gel and oral solid dosage options. Go to www.puracap.com for more information.
Forward-Looking Statement
This news release includes forward-looking statements. Some examples of forward-looking statements include statements regarding the timing and closing of the Epic acquisition, the ability of Humanwell and PuraCap to complete the Epic acquisition considering the various closing conditions, and any assumptions underlying the foregoing. Such statements, based as they are on the current expectations of management, inherently involve numerous risks and uncertainties, known and unknown. Risks and uncertainties include general economic conditions, general conditions in the pharmaceutical industry, changes in the regulatory environment in the jurisdictions in which PuraCap and Humanwell do business, fluctuations in costs, changes to the competitive environment due to consolidation or otherwise, uncertainties as to how Humanwell stockholders may vote on the pending transaction, and the possibility that various closing conditions to the Epic acquisition may not be satisfied or waived (including that a governmental entity may prohibit, delay or refuse to grant approval for the consummation of the acquisition, or that a material adverse effect occurs with respect to Epic). Consequently, actual future results may differ materially from the anticipated results expressed in the forward-looking statements.
Media Contact:
info@puracap.com
908-941-5456
EpiCeram appeared on Channel 2 News Houston and featured in Reader’s Digest article
Piscataway, NJ – Jan 10, 2018 –
EpiCeram appeared “front and center” on Channel 2 News Houston in a TV segment discussing winter skin. Dr. Sherry Ingraham is a regular on-air contributor to the channel’s health segments.
Please click here for more details.
EpiCeram was also mentioned in a recent Reader’s Digest article , ’7 Eczema Treatments Dermatologists Use on Themselves,’ in which prominent Boca Raton, FL dermatologist Dr. Jeffrey Fromowitz declares EpiCeram is “ his go-to for moderate to severe eczema.”
Please click here for more details.