Site Map
© 2024 PuraCap® Pharmaceutical LLC
All Rights Reserved.
This site is only intended for residents of the United States. The products discussed on this website may have different labeling in different countries
PuraCap® Pharmaceutical LLC,
100 Somerset Corp Blvd, Ste 3000
Bridgewater, NJ 08807
Phone: 908.941.5456
Fax: 908.941.5457

Prescription Generic Medications

PuraCap Laboratories develops a wide range of prescription generic medications to meet patient needs for quality and affordability. With our own ANDA development, strategic partnerships, in-licensing agreements, we offer a wide range of high quality prescription generics.
A wide range of PuraCap products are manufactured in our affiliated manufacturing partner, Humanwell Puracap Pharmaceuticals (Wuhan) Co., Ltd, a US FDA cGMP compliant facility that comply with international regulatory standards. Our goal is to put quality first, by building quality through design and a commitment to the highest standards. We strive to exceed USFDA, cGMP standards to assure our customers the quality product they expect will be delivered.
Their experienced manufacturing team, led by a well-recognized technical manufacturing authority in the soft gelatin pharmaceutical capsule industry, is committed to growing our business responsibly – utilizing proven and innovative processes and procedures to produce quality products without compromise. Our manufacturing team is backed by a U.S. executive team with decades of experience in FDA (Federal Drug Administration) regulated prescription drug cGMP quality system management and process validation.
They strive to provide cost-efficiency through manufacture at state-of-the art facilities built to USFDA specifications. Our model for success is to make use of labor as well as manufacturing efficiencies while meeting the highest quality standards. This model sets us apart from the competition. Our customers can be confident in our products—-highest quality at a competitive price.



At PuraCap Caribe we focus on the manufacturing and distribution of high quality generic, solid dosage pharmaceuticals at competitive prices. As our business evolves, our goal remains the same: to enhance existing product lines while maintaining efficient supply chain operations and superior customer service.
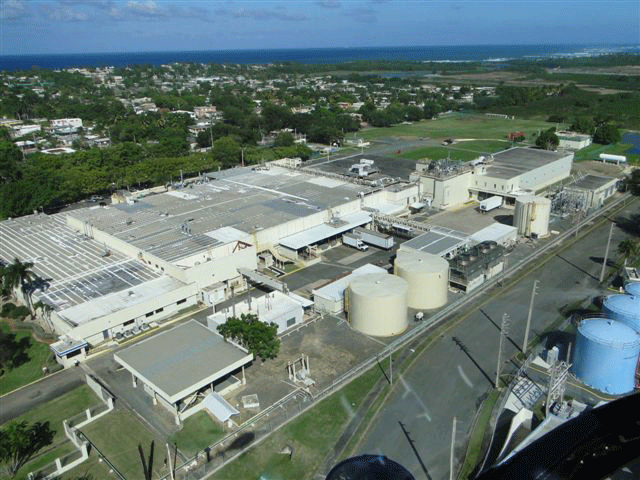
PuraCap Caribe has a state-of- the- art 145,000 sq. ft. FDA approved, cGMP compliant manufacturing facility in Dorado, Puerto Rico. It has over 28,000 sq ft. dedicated to solid dosage manufacturing operations running 24 hours per day seven days per week. This 145,000 sq. ft. facility located on 20 acres has manufactured more than 2 billion tablets per year and the site has an excellent compliance record.
The GMP facility is equipped with a separate full train of manufacturing equipment with several production rooms for Product and Process development, Technology Transfers, Scale up and GMP manufacturing of batches for clinical trials.
PuraCap Laboratories and Puracap Caribe are divisions of PuraCap International




Manufacturing A World of Wellness
Global expertise in product development; producing quality, affordable pharmaceutical and healthcare products.
Click here to join our community
homeHOME











All PuraCap Pharmaceutical prescription
brand products meet our rigorous standards
for quality, accessibility and ease of therapeutic delivery.
PuraCap Laboratories develops & markets prescription generic medications to meet patient needs for quality, efficacy and affordable products.
Our over the counter (OTC) business, Puravation Pharmaceuticals provides many top quality, OTC store brand &
private label products.
With our turn-key services, we can develop Prescription and OTC soft gelatin capsules in our state of the art facility, designed and constructed in accordance with all US FDA cGMP regulatory guidances.









November 16, 2015
South Plainfield, NJ– November 3, 2015 – PuraCap Pharmaceutical LLC
announced today the introduction of a
new product, EpiCeram-L™ Lip Care,
a unique lip care formulation with an
exclusive delivery
July 10, 2014
PuraCap™ Pharmaceutical Introduces
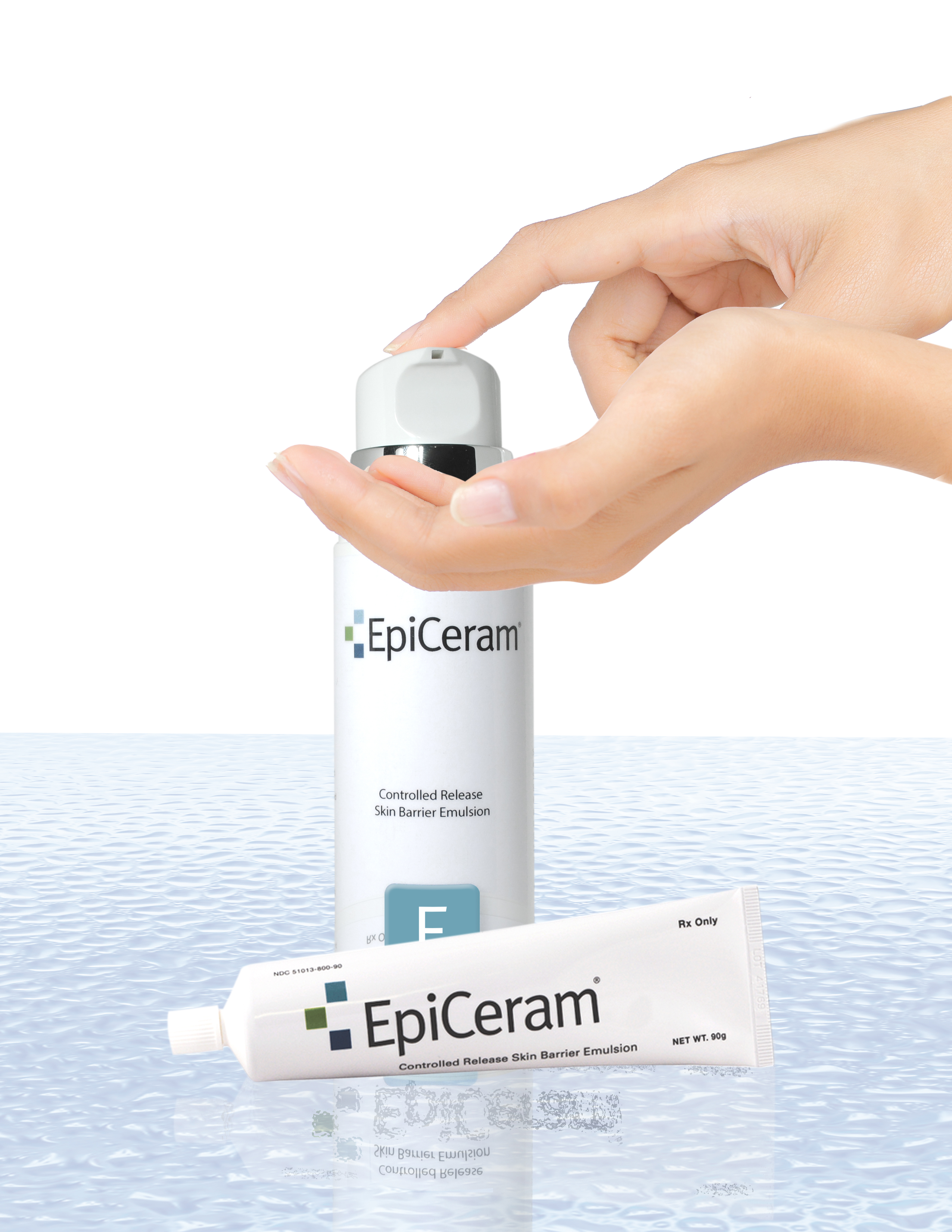
Unique EpiCeram® 225g Airless Pump
EpiCeram® Controlled Release Skin
Barrier Emulsion Now Offers Dosing
Flexibility with 2 Sizes.
November 13, 2015
South Plainfield, NJ–November 11, 2015 – PuraCap Pharmaceutical LLC. is pleased to announce that the US Food and Drug Administration (FDA) has completed a successful inspection of its affiliated
May 19, 2014
Max Baucus, US Ambassador to China visits Wuhan Softgel Capsule Manufacturing Plant in Wuhan China
SOUTH PLAINFIELD, N.J., May 19, 2014 /PRNewswire/ — PuraCap Pharmaceutical today announced


Site Map
© 2024 PuraCap® Pharmaceutical LLC
All Rights Reserved.
This site is only intended for residents of the United States. The products discussed on this website may have different labeling in different countries
PuraCap® Pharmaceutical LLC,
100 Somerset Corp Blvd, Ste 3000
Bridgewater, NJ 08807
Phone: 908.941.5456
Fax: 908.941.5457


Prescription Generic Medications
EpiCeram® is a leading prescription product that has the ability to help repair and heal the skin barrier through a unique mode-of-action different from other medications and eczema treatments. Typically, EpiCeram® is prescribed along with a topical steroid product, but EpiCeram® can also be prescribed alone. Only EpiCeram® contains the skin’s natural level of 3 essential lipids, such as ceramides, cholesterol and free fatty acids, which are reduced in patients with eczema or atopic dermatitis. EpiCeram® is steroid-free, fragrance-free, noncomedogenic1 , paraben-free, and propylene glycol-free.
EpiCeram-L™ Lip Care is the first of its kind, using a patented MultiSal™ Technology an exclusive multi-component controlled-release of important lipids, creating a moist, luxuriant feel.
EpiCeram-L™ Lip Care contains 3 essential lipids ceramides, conjugated linoleic acid (CLA) and cholesterol.
EpiCeram-L™ Lip Care is steroid-free, paraben-free, gluten-free, petrolatum-free, fragrance-free, color-free, and aloe rich to soothe lips on contact. It is ideal for the most severe, dry, cracked lips.




PuraCap Laboratories develops a wide range of prescription generic medications to meet patient needs for quality and affordability. With our own ANDA development, strategic partnerships, in-licensing agreements, we offer a wide range of high quality prescription generics.
A wide range of PuraCap products are manufactured in our affiliated manufacturing partner, Humanwell Puracap Pharmaceuticals (Wuhan) Co., Ltd, a US FDA cGMP compliant facility that comply with international regulatory standards. Our goal is to put quality first, by building quality through design and a commitment to the highest standards. We strive to exceed USFDA, cGMP standards to assure our customers the quality product they expect will be delivered.
Their experienced manufacturing team, led by a well-recognized technical manufacturing authority in the soft gelatin pharmaceutical capsule industry, is committed to growing our business responsibly – utilizing proven and innovative processes and procedures to produce quality products without compromise. Our manufacturing team is backed by a U.S. executive team with decades of experience in FDA (Federal Drug Administration) regulated prescription drug cGMP quality system management and process validation.
They strive to provide cost-efficiency through manufacture at state-of-the art facilities built to USFDA specifications. Our model for success is to make use of labor as well as manufacturing efficiencies while meeting the highest quality standards. This model sets us apart from the competition. Our customers can be confident in our products—-highest quality at a competitive price.




At PuraCap Caribe we focus on the manufacturing and distribution of high quality generic, solid dosage pharmaceuticals at competitive prices. As our business evolves, our goal remains the same: to enhance existing product lines while maintaining efficient supply chain operations and superior customer service.
PuraCap Caribe has a state-of- the- art 145,000 sq. ft. FDA approved, cGMP compliant manufacturing facility in Dorado, Puerto Rico. It has over 28,000 sq ft. dedicated to solid dosage manufacturing operations running 24 hours per day seven days per week. This 145,000 sq. ft. facility located on 20 acres has manufactured more than 2 billion tablets per year and the site has an excellent compliance record.
The GMP facility is equipped with a separate full train of manufacturing equipment with several production rooms for Product and Process development, Technology Transfers, Scale up and GMP manufacturing of batches for clinical trials.


PuraCap Laboratories and Puracap Caribe are divisions of PuraCap International